Redox proteins in endoplasmic reticulum
(In collaboration with Prof. Chichen Wang, IBP)
1. Structural mechanism of substrate binding and modulation for human endoplasmic reticulum protein ER44.
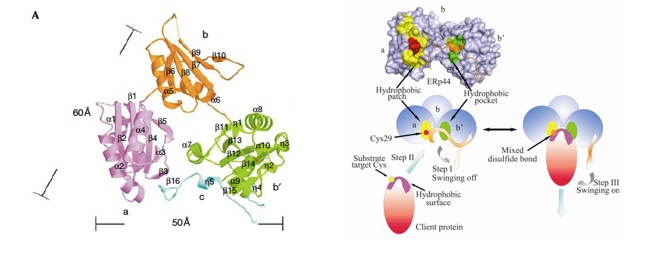
ERp44 mediates thiol-dependent retention in the early secretory pathway, forming mixed disulphides with substrate proteins through its conserved CRFS motif. We solved the crystal structure of ERp44 at a resolution of 2.6A°. Three thioredoxin domains (a,b and b') are arranged in a clover-like structure. A flexible carboxy-terminal tail turns back to the b' and a domains, shielding a hydrophobic pocket in domain b' and a hydrophobic patch around the CRFS motif in domain a. Mutational and functional studies indicate that the C-terminal tail gates the CRFS area and the adjacent hydrophobic pocket, dynamically regulating protein quality control.
Reference:
Wang, L., Wang, L., Vavassori, S., Li, S., Ke, H., Anelli, T., Degano, M., Ronzoni, R., Sitia, R., Sun, F.* & Wang, C. C.* (2008) Crystal structure of human ERp44 shows a dynamic functional modulation by its carboxy-terminal tail, EMBO Rep.9. 642-7.
2. Structural insights into the peroxidase activity and inactivation of human peroxiredoxin 4.
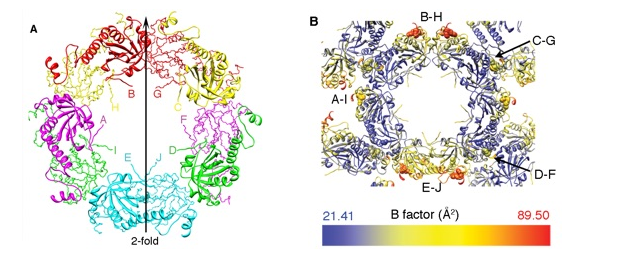
Prx4 (peroxiredoxin 4) is the only peroxiredoxin located in the ER (endoplasmic reticulum) and a proposed scavenger for H2O2. In this work we presented crystal structures of human Prx4 in three different redox forms and characterized the reaction features of Prx4 with H2O2. Prx4 exhibits a toroid-shaped decamer constructed of five catalytic dimers. Structural analysis revealed conformational changes around helix α2 and the C-terminal reigon with a YF motif from the partner subunit, which are required for inter-chain disulfide formation between Cys87 and Cys208. a critical step of the catalysis. The structural explanation for the restricting role of the YF motif on the active site dynamics is provided in detail. Prx4 has a high reactivity to H2O2. but is susceptible to over-oxidation and consequent inactivation by H2O2. Either deletion of the YF motif or dissociation into dimers decreased the susceptibility of Prx4 to over-oxidation by increasing the flexibility of Cys87.
Reference:
Wang Xi, Wang Likun, Wang Xi's, Sun Fei* and Wang Chih-chen*. (2011), Structural insights into the peroxidase activity and inactivation of human peroxiredoxin 4. Biochem. J., 441: 113-118.
附件下载: