Structural and functional study of group II chaperonin
(In collaboration with Prof. Zhiyang Dong, Institute of Microbiology, CAS)
1. Different symmetry and limited peptides refolding activity of the thermosomes from acidothermophilic archaea Acidianus tengchongensis S5T
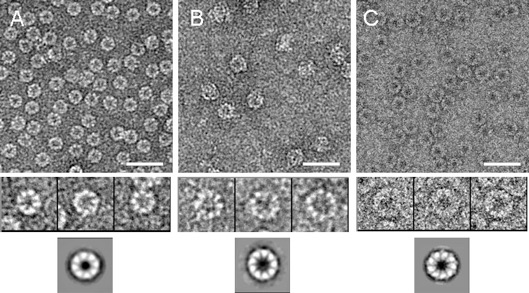
Recombinant thermosomes from Acidianus tengchongensis strain S5T were purified to homogeneity and assembled in vitro either into homo-oligomer rATcpnα/rATcpnβ or hetero-oligomer rATcpnαβ. They were analyzed by electron microscopy and image analysis to determine the symmetries of these particles. rATcpnα presents as 8-fold symmetry while both rATcpnβ and rATcpnαβ adopt 9-fold symmetry. rATcpnαβ contain the α and β subunits in a 1:2 ratio. All of them prevent the irreversible inactivation of yeast alcohol dehydrogenase at 55 °C and completely prevent the formation of aggregates during thermal inactivation of citrate synthase at 45 °C. They all show trace ATP hydrolysis activity. Furthermore, rATcpnβ sequestered the chemically fully-denatured substrates (GFP and thermophilic malic dehydrogenase) without refolding them in ATP-dependent manner in vitro. This property is quite similar to chaperonins from Sulfolobus tokodaii and Sulfolobus acidocaldarius reported previously. This is consistent with the slow growth rates of these archaeas during surviving in their native environment.
Reference:
Wang L., Hu Z., Luo Y., Huo Y., Ma Q., He Y., Zhang Y.,Sun F.*, Dong Z.* (2010), Distinct symmetry and limited peptide refolding activity of the thermosomes from the acidothermophilic archaea Acidianus tengchongensis S5T, Biochem Biophys Res Commun, 393: 228-234.
2. Open structure of 9-fold thermosome rATcpnβ from Acidianus tengchongensis S5T
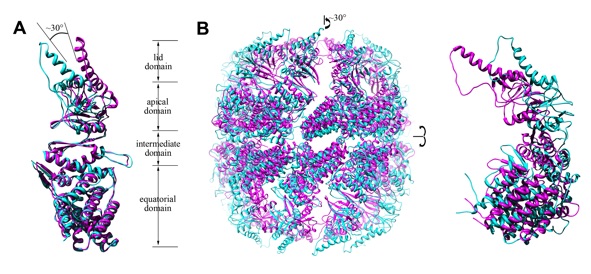
Thermosomes are group II chaperonins responsible for protein refolding in an ATP-dependent manner. Little is known regarding the conformational changes of thermosomes during their functional cycle due to lack of high-resolution structure in open state. Here we report the first complete crystal structure of thermosome (rATcpnβ) in open state from Acidianus tengchongensis. There is a ~30° rotation of the apical and lid domains compared to the previous closed structure. Besides, the structure reveals a conspicuous hydrophobic patch in the lid domain and residues locating in this patch are conserved across species. Both the closed and open forms of rATcpnβ were also reconstructed by electron microscopy (EM). Structural fitting revealed the detailed conformational change from open to closed state. Structural comparison as well as protease K digestion indicated only ATP binding without hydrolysis does not induce chamber closure of thermosome.
Reference:
Hu YW, Hu ZJ, Zhang K, Wang L, Zhai Y, Zhou QJ, Lander G, Zhu J, He YZ, Pang XY, Xu W, Bartlam M, Dong ZY*, Sun F.*. (2010). Crystal structure of group II chaperonin in the open state. Structure, 18(10): 1270-1279.
3. Substrate binding property of the thermosome rATcpnβ from Acidianus tengchongensis S5T

The residues associated with substrate binding in Group I chaperonin GroEL are hydrophobic. However, the corresponding residues in group II chaperonin ATcpnβ from Acidianus tengchongensis are hydrophilic.When we mutate these hydrophilic residues in ATcpnβ to hydrophobic residues, i.e. residues 236 from G to Y, 237 from M to F, 288 from D to L, 295 from A to V, 313 from A to V and 314 from K to V. They enhanced the inhibitory effect of ATcpnβ on the refolding of acid-denatured GFP and the inhibition activity of citrate synthase (CS) thermal aggregation.We suggest that hydrophobic interaction contribute more to peptides binding affinity both in group I and group II chaperonin. Chaperonins have been proved to have ATP-dependent peptides refolding ability. However, it is still unclear whether peptides binding ability of chaperonins is ATP-dependent. We use Surface plasma resonance (SPR) analysis to test chaperonin binding ability to different peptides with different denaturing level. Our assays revealed that ATcpnβ could capture guanidine hydrochloride denatured malate dehydrogenase in a Mg2+/ATP independent manner while it bind thermal aggregated citrate synthase or lysozyme in a Mg2+/ATP dependent manner. We demonstate that chaperonin conformatinal changes induced by Mg2+/ATP to expose more hydrophobic surface is required for chaperonin capturing thermal aggregated peptides which has larger hydrophobic surfaces.
Reference:
Wang L., Zhang K., Fan Z., Dong Z.*, Sun F.* (2011), Substrate binding properties of thermosome ATcpn-beta from Acidianus Tengchongensis. Progress in Biochemistry and Biophysics, 38(2):151-158.
4.Determinants of thermal stability of group II chaperonins from Acidianus tengchongensis S5T (in collaboration with Klaus Schulten, UIUC)

Group II chaperonins, which assemble as double-ring complexes, assist in the refolding of nascent peptides or denatured proteins in an ATP-dependent manner. The molecular mechanism of group II chaperonin assembly and thermal stability is yet to be elucidated. Here, we selected the group II chaperonins (cpn-α and cpn-β), also called thermosomes, from Acidianus tengchongensis and investigated their assembly and thermal stability. We found that the binding of ATP or its analogs contributed to the successful assembly of thermosomes and enhanced their thermal stabilities. Cpn-β is more thermally stable than cpn-α, while the thermal stability of the hetero thermosome cpn-αβ is intermediate. Cryo-electron microscopy reconstructions of cpn-α and cpn-β revealed the interwoven densities of their non-conserved flexible N/C-termini around the equatorial planes. The deletion or swapping of their termini and pH-dependent thermal stability assays revealed the key role of the termini electrostatic interactions in the assembly and thermal stability of the thermosomes.
Reference:
Zhang K., Wang L., Liu Y., Chan K.Y., Pang X., Schulten K., Dong Z. andSun F.* (2013), Flexible interweaved termini determine the thermal stability of thermosomes. Protein Cell, 4(6): 432-444.
附件下载: