Structural insights into the intrinsic self-assembly of Par-3 N-terminal domain by cryo-electron microscopy and helical reconstruction
(In collaboration with Prof. Wei Feng , IBP and Prof. Mingjie Zhang, HKUST)
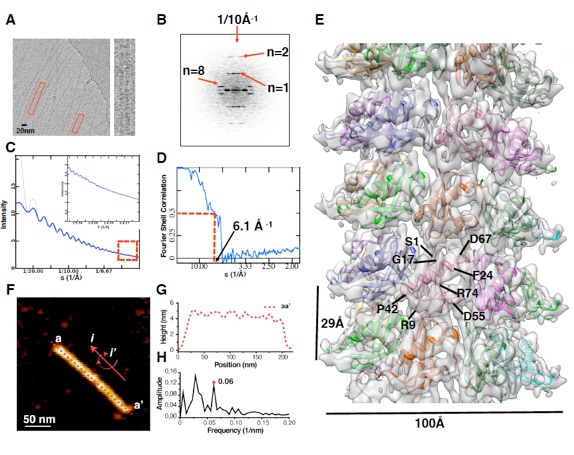
Par-3. the central organizer of the Par-3/Par-6/aPKC complex, is a multimodular scaffold protein that is essential for cell polarity establishment and maintenance. The N-terminal domain (NTD) of Par-3 is capable of self-association to form filament-like structures, although the underlying mechanism is poorly understood. Here, we determined the crystal structure of Par-3 NTD and solved the filament structure by cryo-electron microscopy. We found that an intrinsic “front-to-back” interaction mode is important for Par-3 NTD self-association and that both the lateral and longitudinal packing within the filament are mediated by electrostatic interactions. Disruptions of the lateral or longitudinal packing significantly impaired Par-3 NTD self-association and thereby impacted the Par-3-mediated epithelial polarization. We finally demonstrated that a Par-3 NTD-like domain from histidine ammonia-lyase also harbors a similar self-association capacity. This work unequivocally provides the structural basis for Par-3 NTD self-association and characterizes one type of protein domain that can self-assemble via electrostatic interactions.
Reference:
Zhang Y., Wang W., Chen J., Zhang K., Gao F., Gao B., Zhang S., Dong M., Besenbacher F., Gong W., Zhang M., Sun F.* and Feng W.* (2013), Structural insights into the intrinsic self-assembly of Par-3 N-terminal domain.Structure, 21(6):997-1006.
附件下载: