Dimerization interface of 3-hydroxyacyl-CoA dehydrogenase tunes the formation of its catalytic intermediate
(In collaboration with Prof. Jun Fan, City University of HongKong and Prof. Yinhua Jin, Jilin University)
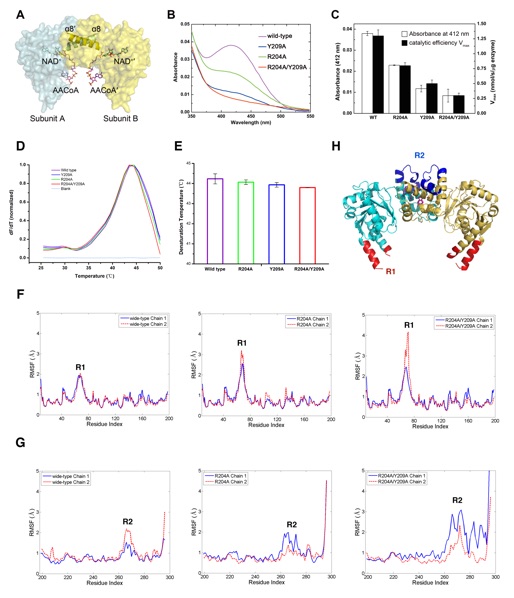
3-hydroxyacyl-CoA dehydrogenase (HAD, EC 1.1.1.35) is a homodimeric enzyme localized in the mitochondrial matrix, which catalyzes the third step in fatty acid β-oxidation. The crystal structures of human HAD and subsequent complexes with cofactor/substrate enabled better understanding of HAD catalytic mechanism. However, numerous human diseases were found related to mutations at HAD dimerization interface that is away from the catalytic pocket. The role of HAD dimerization in its catalytic activity needs to be elucidated. Here, we solved the crystal structure of Caenorhabditis elegans HAD (cHAD) that is highly conserved to human HAD. Even though the cHAD mutants (R204A, Y209A and R204A/Y209A) with attenuated interactions on the dimerization interface still maintain a dimerization form, their enzymatic activities significantly decrease compared to that of the wild type. Such reduced activities are in consistency with the reduced ratios of the catalytic intermediate formation. Further molecular dynamics simulations results reveal that the alteration of the dimerization interface will increase the fluctuation of a distal region (a.a. 60-80) that plays an important role in the substrate binding. The increased fluctuation decreases the stability of the catalytic intermediate formation, and therefore the enzymatic activity is attenuated. Our study reveals the molecular mechanism about the essential role of the HAD dimerization interface in its catalytic activity via allosteric effects.
Reference:
Xu YZ., Li H., Jin YH., Fan J.* and Sun F.* (2014), Dimerization interface of 3-hydroxyacyl-CoA dehydrogenase tunes the formation of its catalytic intermediate. PLOS one. 9(4): e95965. doi:10.1371/journal.pone.0095965.
附件下载: