Molecular mechanism of the enzyme promiscuity from an aromatic prenyltransferase
(In collaboration with Prof. Jungui Dai, Chinese Academy of Medical Science and Peking Union Medical College)
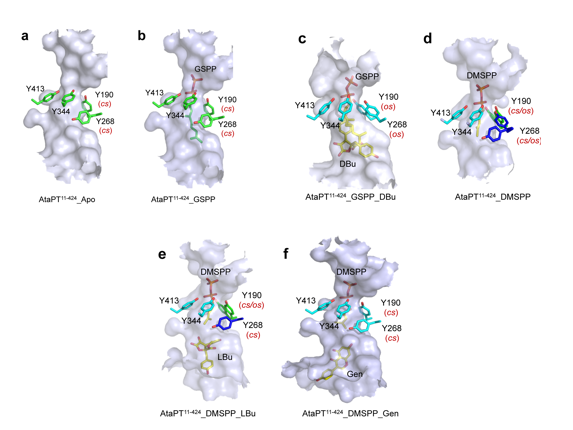
Conformational dynamics of the tyrosine shield and various acceptor binding sites of AtaPT. The substrate-binding pockets, tyrosine shields, prenyl donors and aromatic acceptors from the structures of (a) apo-AtaPT, (b) AtaPT-GSPP, (c) AtaPT-GSPP-DBu, (d) AtaPT-DMSPP, (e) AtaPT-DMSPP-LBu, and (f) AtaPT-DMSPP-Gen, shown in surfaces and sticks. Residue Y190 and Y268 show two conformations with the closed state (cs) and the alternative open state (os).
Aromatic prenyltransferases (aPTases) transfer prenyl moieties from isoprenoid donors to various aromatic acceptors, and some of them have the rare property of extreme enzymatic promiscuity toward both a variety of prenyl donors and a large diversity of acceptors. In this study, we discovered a novel aPTase, AtaPT, from Aspergillus terreus that exhibits unprecedented promiscuity toward diverse aromatic acceptors and prenyl donors and also yields products with a range of prenylation patterns. Systematic crystallographic studies revealed various discrete conformations for ligand binding with the donor-dependent acceptor specificity and multiple binding sites within a spacious hydrophobic substrate-binding pocket. Further structure-guided mutagenesis of active sites at the substrate-binding pocket is responsible for altering the specificity and promiscuity toward substrates and the diversity of product prenylations. Our study reveals the molecular mechanism underlying the promiscuity of AtaPT and immediately suggests an efficient protein engineering strategy to generate novel prenylated derivatives in drug discovery applications.
Reference:
Chen R., Gao B., Liu, X., Ruan F., Zhang Y., Lou J., Feng K., Wunsch C., Li S.M., Dai J.* and Sun F.* (2016), Molecular insights into the enzyme promiscuity of an aromatic prenyltransferase. Nature Chemical Biology (Advanced online at Dec. 19, 2016), doi: 10.1038/nchembio.2263.
附件下载: