Cryo-EM structure and electrophysiological characterization of ALMT from Glycine max reveal a previously uncharacterized class of anion channels
Anion fluxes in plants play a fundamental role in cell signal transduction and volume regulation. Aluminum-activated malate transporters (ALMTs) form an anion channel family that plays essential roles in diverse functions in plants Arabidopsis ALMT12, also named QUAC1 (quick anion channel 1), regulates stomatal closure in response to environmental stimuli. However, the molecular basis of ALMT12/QUAC1 activity remains elusive. In 2022, Chen Lab published the article titled Cryo-EM structure and electrophysiological characterization of ALMT from Glycine max reveal a previously uncharacterized class of anion channels in Science Advances, which uncovers the molecular basis for the gating and modulation of the ALMT12/QUAC1 anion channel.
In the article, the authors describe the cryo-EM structure of ALMT12/QUAC1 from Glycine max at 3.5 A resolution. GmALMT12/QUAC1 is a symmetrical dimer, forming a single electropositive T-shaped pore across the membrane. The transmembrane and cytoplasmic domains are assembled into a twisted two-layer architecture, with their associated dimeric interfaces nearly perpendicular.
GmALMT12/QUAC1-mediated currents display rapid kinetics of activation/deactivation and a bell-shaped voltage dependency, reminiscent of the rapid (R)-type anion currents. The structural and functional analyses reveal a domain-twisting mechanism for malate-mediated activation.
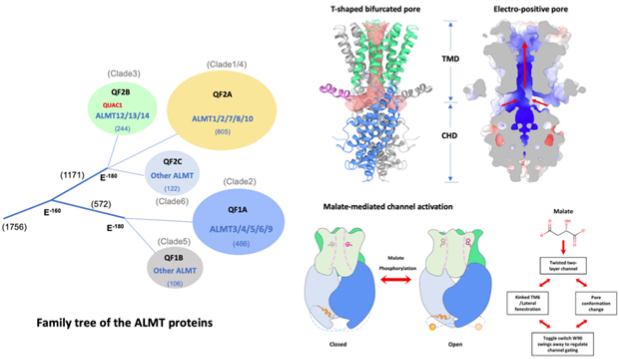
Together, the study uncovers the molecular basis for a previously uncharacterized class of anion channels and provides insights into the gating and modulation of the ALMT12/QUAC1 anion channel.
Link: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8890709/
附件下载: