Structural study of autotransporter from B. pertussis
1. Crystal structure of the transmembrane beta-domain of Bordetella serum-resistance killing protein A, revealing the mechanism of the secretion of its passenger domain.
(In collaboration with Prof. Neil Isaacs, University of Glasgow, UK )

Whooping cough (pertussis) is a highly contagious, acute respiratory illness of humans caused by the gram-negative bacterial pathogen Bordetella pertussis. The autotransporter (AT) BrkA is an important B. pertussis virulence factor that confers serum resistance and mediates adherence. Here we present the crystal structure of B. pertussis BrkA β domain at 3? resolution. Special features are a hairpin-like structure formed by the external loop L4, which is observed fortuitously sitting inside the pore of the crystallographic adjacent β domain; and a previously undiscovered hydrophobic cavity formed by patches on the loop L4 and β-strands S5 and S6. This adopts an ubiquitous structure characteristic of all AT β domains. Mutagenesis studies have demonstrated that the hairpin-like structure and hydrophobic cavity are crucial for BrkA passenger domain (virulence effector) translocation. This structure helps in understanding the molecular mechanism of AT assembly and secretion and provides a potential target for anti-pertussis drug design.
Reference:
Zhai Y., Zhang K., Huo Y., Zhu Y., Zhou Q., Lu J., Black I., Pang X., Roszk A.W., Zhang X., Isaacs N.W., Sun F.* (2011), Autotransporter passenger domain secretion requires a hydrophobic cavity at the extracellular entrance of the beta-domain pore. Biochem. J. , 435:577-587.
2. Development of a bacterial surface display system (BrkAutoDisplay) based on the structure of BrkA beta domain.
(In collaboration with Prof. Ganggang Wang, Chengdu Institute of Biology, Chinese Academy of Sciences)
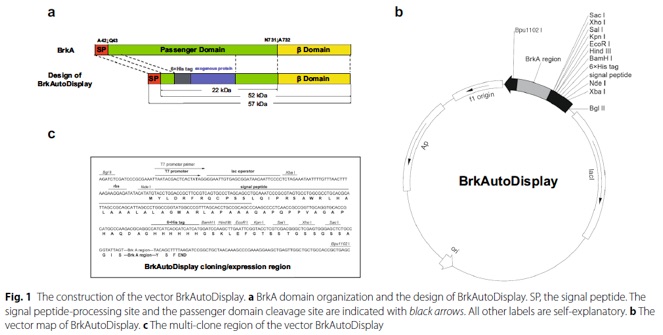
Bacterial surface display technique enables the exogenous proteins or polypeptides displayed on the bacterial surface, while maintaining their relatively independent spatial structures and biological activities. The technique makes recombinant bacteria possess the expectant functions, subsequently, directly used for many applications. Many proteins could be used to achieve bacterial surface display, among them, autotransporter, a member of the type V secretion system of gram-negative bacteria, has been extensively studied because of its modular structure and apparent simplicity. However, autotransporter has not been widely used at present due to lack of a convenient genetic vector system. With our recently characterized autotransporter BrkA (Bordetella serum-resistance killing protein A) from Bordetella pertussis, we are aiming to develop a new autotransporter-based surface display system named as BrkAutoDisplay for potential wide application. BrkAutoDisplay is a convenient system to host exogenous genes. In our test, this system is good to efficiently display various proteins on the outer membrane surface of E. coli., including green fluorescent protein (GFP), various enzymes and single chain antibody. Moreover, the displayed GFP possesses green fluorescence, the enzymes CotA, EstPc and PalA exhibit catalytic activity 0.12 mU, 6.88 mU and 0.32 mU (per 5.2×108 living bacteria cells) respectively, and the single chain antibody fragment (scFv) can bind with its antigen strongly. Our results indicate that BrkAutoDisplay system is worthy of further study for industrial applications.
Reference:
Sun F. (Sun Fang), Pang X., Xie T., Zhai Y., Wang G.G.* and Sun F.* (2015), BrkAutoDisplay: functional display of multiple exogenous proteins on the surface of Escherichia coli by using BrkA autotransporter. Microbial Cell Factories. 14: 129. doi: 10.1186/s12934-015-0316-3.
附件下载: