Structure and function of ryanodine receptor 1 (RyR1)
(In collaboration with Prof. Chang-cheng Yin, Peking University)
1. Structure of RyR1 in Ca2+ activated state.
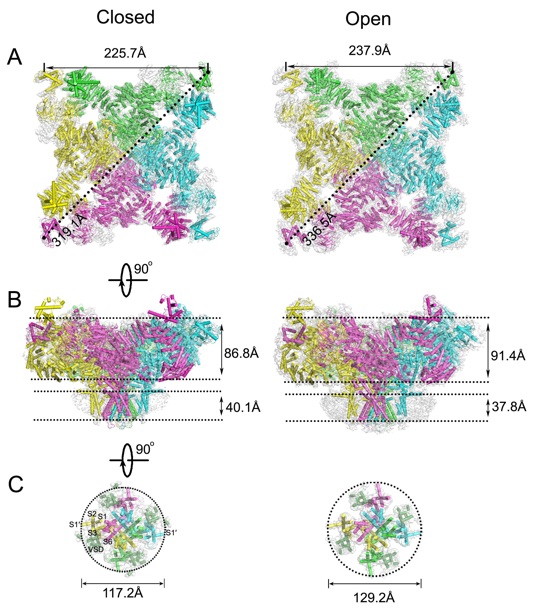
Structural comparison between apo/closed and activated/open RyR1s. Structure models (colored cylinders and lines) of closed-state RyR1 and open-state RyR1 are superimposed on the cryo-EM maps (semi-transparent grey) in top (A), side (B), and bottom (C) views. In the top views, the side and diagonal dimensions of the cytoplasmic assembly of each state are labeled. In the side views, the heights of the cytoplasmic assembly and the transmembrane region of each state are marked. In the bottom views, only the transmembrane region, including helices S1~S6, the linker helix S4-S5, and extension helix S1’, is displayed with the diameter labeled. See also Figures S1, S2, S3, and S4.
Ryanodine receptors (RyRs) are a class of giant ion channels composed of four subunits with molecular masses over 2.2 mega-Daltons. These channels mediate calcium signaling in a variety of cells, with their role in muscle cells during excitation-contraction coupling being especially well known. Since more than 80% of the RyR protein is folded into the cytoplasmic assembly and the remaining residues form the transmembrane domain, it has been hypothesized that the activation and regulation of RyR channels occurs through an as yet uncharacterized long-range allosteric mechanism. Here, we report the characterization of an open-state RyR1 structure activated solely by a Ca2+ by cryo-electron microscopy. The structure has an overall resolution of 4.9 ? and a resolution of 4.2 ? for the core region. In comparison with the previously determined apo/closed-state structure of RyR1, we observed long-range allosteric gating of the channel upon Ca2+ activation and the structural basis for RyRs’ broad-spectrum ion conductivity. Our work provides in-depth insight into the molecular mechanisms of channel gating and the regulation of RyRs.
Reference:
Wei R., Wang X., Zhang Y., Mukherjee S., Zhang L., Chen Q., Huang X., Jing S., Liu C., Li S., Wang G., Xu Y., Zhu S., Williams A., Sun F.* and Yin C.C.* (2016), Structural insights into Ca2+ -activated long-range allosteric channel gating of RyR1. Cell Research 26: 977-994. doi: 10.1038/cr.2016.99
附件下载: