Structure of Hyperthermophilic Respiratory Complex III from Aquifex aeolicus
(In collaboration with Prof. Hartmut Michel from Max Planck institute, Germany)
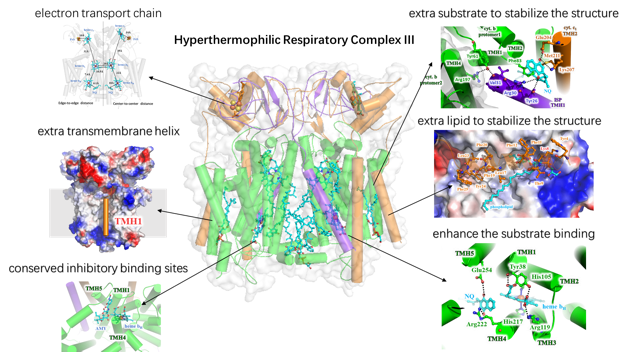
Cellular respiration complexes convert redox energy into a transmembrane electrochemical proton gradient which is used to synthesize adenosine triphosphate (ATP) or to transport various substances. In this process, the cytochrome bc1 complex (also known as complex III) plays a key role by catalyzing the electron transfer from quinols to cytochrome c simultaneously transporting protons across the membrane according to the “Q-cycle” mechanism. At present, various structures of cytochrome bc1 complexes from vertebrates, yeast, and α-proteobacteria are available. Three conserved core subunits are always present, namely cytochrome b (cyt. b) with the cofactor heme bL and heme bH, cytochrome c1 (cyt. c1) with cofactor heme c1, and a Rieske iron-sulfur protein (ISP) with a binuclear iron sulfur cluster (2Fe-2S). However, our knowledge of cytochrome bc1 complex structures has been so far limited to those from mesophilic species, so little information is available on its structures from thermophiles, which hinders our understanding of its unique thermal stability allowing maintenance of the electron transfer reaction under extreme conditions.
Aquifex aeolicus is a hyperthermophilic chemoautotrophic ε-proteobacterium with adaptive growth temperatures in the range of 85-95°C. As one of the most hyperthermophilic bacteria known, A. aeolicus is thought to be one of the oldest bacterial species. and its respiratory chain complexes usually use hydrogen as the primary electron donor and oxygen as electron acceptor in order to provide energy for metabolism. In particular, its cytochrome bc1 complex uses a naphthoquinone derivative 2-VI,VII-tetrahydromultiprenyl-1,4-naphthoquinone (NQ), as special substrate for electron transfer, with NQ being reduced to NQH2 by electrons from hydrogen oxidation in previous reactions. Moreover, this complex has a significantly increased stability at extremely high temperatures.
In this work, we solved the 3.3 ? resolution structure of respiratory chain complex III from A. aeolicus in both natural state and inhibited state, using cryo-EM single particles analysis. It’s the first high-resolution three-dimensional structure of respiratory chain complex III from thermophilic species. By sequence alignment and structural analysis, we identified a series of novel structural characteristic to significantly enhance the stability of the protein complex, and found a number of special residues with an extra transmembrane helix in thermophiles to stabilize the ligands and subunits. Moreover, the complex III has hold an extra quinone and phospholipid molecules inside the protein, which further enhance the stability of the protein complex on the cell membrane. These finding reveals the structural basis of complex to remain stable in extreme environment, and provides theoretical basis and experimental data for future study of other proteins’ stability in extreme environment.
Reference:
Zhu G., Zeng H., Zhang S., Juli J., Pang X., Hoffmann J., Zhang Y., Morgner N., Zhu Y.*, Peng G.*, Michel H.* and Sun F.* (2019) A 3.3 A-resolution structure of hyperthermophilic respiratory complex III reveals the mechanism of its thermal stability. Angew Chem Int Ed Engl. 59 (1): 343-351. doi: 10.1002/anie.201911554.
附件下载: