Cryo-electron tomography reveals the packaging pattern of RuBisCOs in Synechococcus β-carboxysome
Carboxysomes are large self-assembled microcompartments that serve as the central machinery of a CO2-concentrating mechanism (CCM). Biogenesis of carboxysome requires the fine organization of thousands of individual proteins; however, the packaging pattern of internal RuBisCOs remains largely unknown. Here we purified the intact β-carboxysomes from Synechococcus elongatus PCC 7942 and identified the protein components by mass spectrometry. Cryo-electron tomography combined with subtomogram averaging revealed the general organization pattern of internal RuBisCOs, in which the adjacent RuBisCOs are mainly arranged in three distinct manners: head-to-head, head-to-side, and side-by-side. The RuBisCOs in the outermost layer are regularly aligned along the shell, the majority of which directly interact with the shell. Moreover, statistical analysis enabled us to propose an ideal packaging model of RuBisCOs in the β-carboxysome. These results provide new insights into the biogenesis of β-carboxysomes and also advance our understanding of the efficient carbon fixation functionality of carboxysomes.
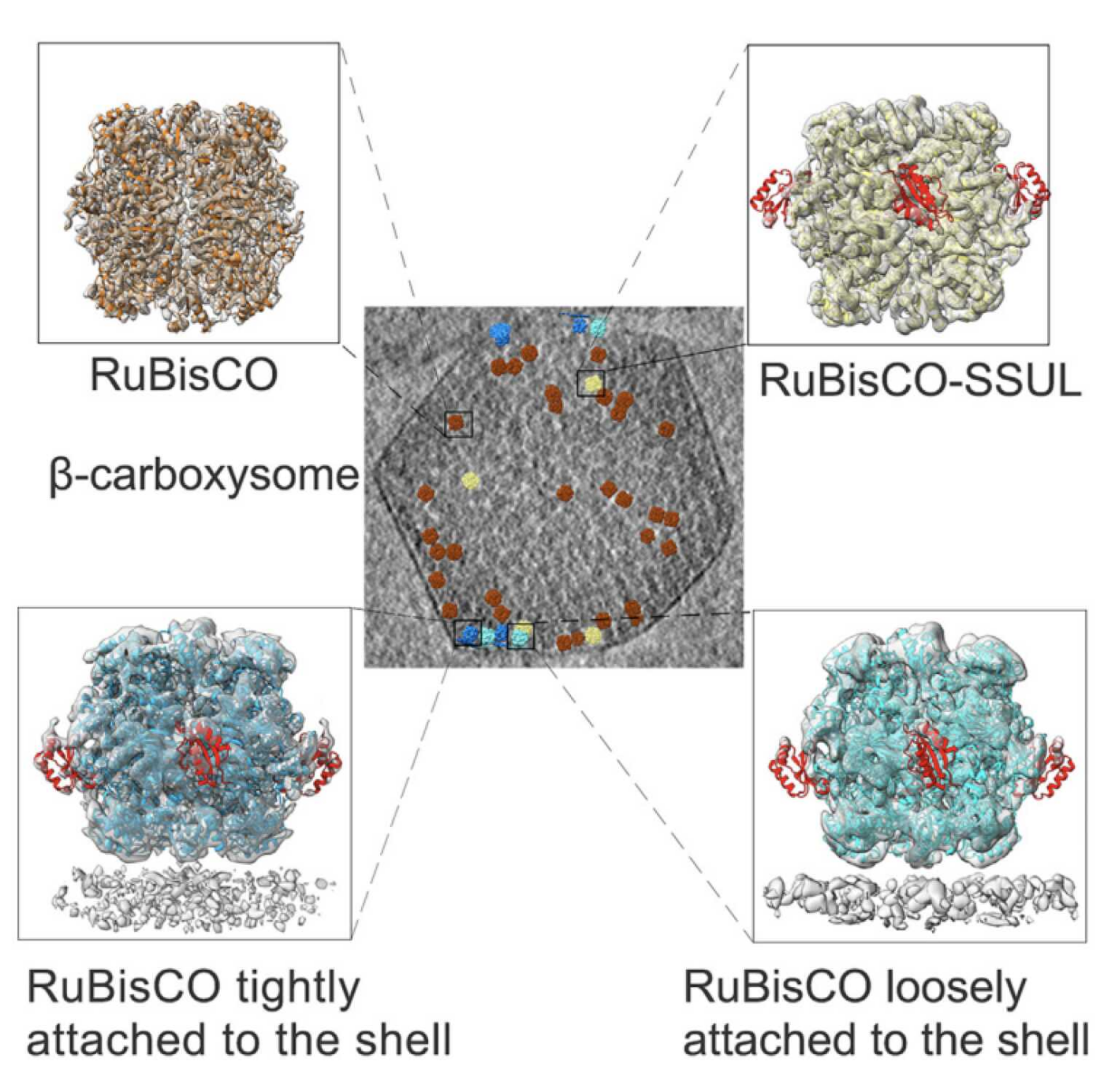
RuBisCOs are densely packed in b-carboxysomes in a lattice-like arrangement
附件下载: