2000.09 - 2004.06 武汉大学生命科学学院,生物学基地班,理学学士
2004.09 - 2009.06 中国科学院生物物理所,理学博士(导师:王志珍院士)
2009.07 - 2011.12 中国科学院生物物理所,助理研究员
2011.02 - 2011.07 意大利圣拉菲尔科学研究所,中科院访问学者
2012.01 - 2019.12 中国科学院生物物理所,副研究员
2020.01 - 至今 中国科学院生物物理所,研究员
2020.01 - 至今 中国科学院大学,岗位教师
2022.04至今 国际学术期刊《Traffic》编委
2021.08-2025.08 中国生物物理学会亚细胞结构与功能分会副秘书长
2021 中国科学院青年创新促进会优秀会员
2016 中国科学院青年创新促进会会员
2015.01-2018.12 中国生理学会应激生理学专业委员会委员
2009 第21届国际生物化学与分子生物学大会青年科学家奖
2009 中国科学院朱李月华奖
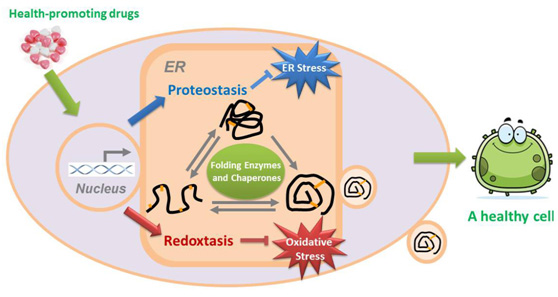
内质网是真核细胞内膜系统的最大组成部分,是蛋白质合成加工的工厂。内质网拥有一整套包括折叠酶和分子伴侣在内的"质量控制"系统,维持内质网的蛋白质稳态。内质网的一个重要功能就是提供合适的氧化还原环境,保证含二硫键的蛋白质(许多分泌蛋白和膜蛋白)正确折叠,即蛋白质氧化折叠。内质网氧化还原稳态与人类健康的关系近年来引人关注。王磊在生物物理所师从王志珍院士攻读博士学位期间开始体外重构真核细胞内质网氧化折叠系统,博士毕业留所工作后拓展到内质网氧化还原稳态与人类健康研究。迄今已在本领域国际高水平学术期刊发表研究论文40余篇,相关工作多次受邀在国际国内学术会议作报告。
近期主要工作进展:
1. 蛋白质氧化折叠系统的工作机制
内质网巯基氧化酶Ero1,蛋白质二硫键异构酶PDI和内质网过氧化物酶GPx7等构成了催化蛋白质氧化折叠的主要通路。细胞在维持二硫键形成的高效性的同时,需要避免其副产物过氧化氢的过度累积。我们的系统研究揭示了Ero1/PDI/GPx7三元系统的精密调节机制是如何保证二硫键形成的高效性和安全性,提出了高等真核细胞内质网蛋白质氧化折叠系统较为完整的工作模型。针对蛋白质氧化折叠的研究不仅有助于深入理解蛋白质折叠的基本规律,也有助于设计更高效的"细胞工厂"用于生产蛋白质药物。
2. 分泌途径氧化还原稳态与人类健康
近年来的研究逐渐认识到,内质网氧化还原稳态的失衡与许多重大疾病密切相关。我们的研究揭示了内质网Ero1/PDI/GPx7蛋白质氧化折叠系统在衰老、癌症和心血管疾病中的重要作用。最新的研究发现细胞表面也存在蛋白质氧化折叠系统,并且参与调控许多细胞生命活动(如血小板活化、病毒入侵和炎症应答等)。针对分泌途径氧化还原稳态的机制研究,将有助于开发新的干预手段,为相关疾病的防治提供新的策略。
3. 分泌途径激酶与人类疾病
以Fam20C为代表的分泌途径激酶是近年来发现的一类新型激酶家族。我们的研究发现Fam20C是一个受到蛋白酶剪切加工调节的高尔基体膜蛋白,这一调控机制在生物矿化过程中起到重要作用。我们还揭示了在应激状态下,Fam20C通过磷酸化内质网关键酶分子来快速精准地调节内质网稳态的新机制。鉴于分泌途径激酶的底物多样性,针对其调节机制和生理功能的研究将为相关疾病的防治提供新的思路。
1. Zhu Y, Wang L, Li J, Zhao Y, Yu X, Liu P, Deng X, Liu J, Yang F, Zhang Y, Yu J, Lai L, Wang C, Li Z*, Wang L*, Luo T* (2024) Photoaffinity labeling coupled with proteomics identify PDI-ADAM17 module is targeted by (-)-vinigrol to induce TNFR1 shedding and ameliorate rheumatoid arthritis in mice. Cell Chem Biol doi.org/10.1016/j.chembiol.2023.10.003.
2. Liu P, Hu J, Wang L* (2024) SARS-CoV-2 ORF8 does not function in the nucleus as a histone mimic. Protein & Cell 15: 79-82.
3. Cheng F, Ji Q, Wang L, Wang CC, Liu GH*, Wang L* (2023) Reducing oxidative protein folding alleviates senescence by minimizing ER-to-nucleus H2O2 release. EMBO Rep 24: e56439.
(Selected as a cover story on EMBO Reports)
4. Liu P, Wang X*, Sun Y, Zhao H, Cheng F, Wang J, Yang F, Hu J, Zhang H, Wang CC, Wang L* (2022) SARS-CoV-2 ORF8 reshapes the ER through forming mixed disulfides with ER oxidoreductases. Redox Biol 54:102388.
5. Wang L*, Wang X, Lv X, Jin Q, Shang H, Wang CC, Wang L* (2022) The extracellular Ero1α/PDI electron transport system regulates platelet function by increasing glutathione reduction potential. Redox Biol 50: 102244
6. Qiao X, Zhang Y, Ye A, Zhang Y, Xie T, Lv Z, Shi C, Wu D, Chu B, Wu X, Zhang W, Wang P, Liu GH, Wang CC, Wang L*, Chen C* (2022) Reductive stress in the endoplasmic reticulum caused by Ero1α S-nitrosation accelerates senescence. Free Radic Biol Med 180: 165-178.
7. Fan F, Zhang Q, Zhang Y, Huang G, Liang X, Wang CC, Wang L*, Lu D* (2022) Two protein disulfide isomerase subgroups work synergistically in catalyzing oxidative protein folding. Plant Physiol 188: 241-254.
8. Chen X, Zhang J, Liu P, Wei Y, Wang XE, Xiao J, Wang CC, Wang L* (2021) Proteolytic processing of secretory pathway kinase Fam20C by site-1 protease promotes biomineralization. Proc Natl Acad Sci U S A 118: e2100133118.
9. Yan Y, Wu X, Wang P, Zhang S, Sun L, Zhao Y, Zeng GY, Liu B, Xu G, Liu H, Wang L*, Wang X* and Jiang C* (2020) Homocysteine promotes hepatic steatosis by activating the adipocyte lipolysis in a HIF1α-ERO1α-dependent oxidative stress manner. Redox Biol 37: 101742.
10. Yu J, Li T, Liu Y, Wang X, Zhang J, Wang Xe, Shi G, Lou J, Wang L, Wang CC, Wang L* (2020) Phosphorylation switches protein disulfide isomerase activity to maintain proteostasis and attenuate ER stress. EMBO J 39: e103841
(Highlighted by EMBO J 'News & Views'; Recommended by F1000Prime)
11. Fan F, Zhang Y, Huang G, Zhang Q, Wang CC, Wang L*, Lu D* (2019) AtERO1 and AtERO2 exhibit differences in catalyzing oxidative protein folding in the ER. Plant Physiol 180:2022-2033.
12. Zhang Y, Li T, Zhang L, Shangguan F, Shi G, Wu X, Cui Y, Wang Xe, Wang X, Liu Y, Lu B, Wei T, Wang CC, Wang L* (2019) Targeting the functional interplay between endoplasmic reticulum oxidoreductin-1α and protein disulfide isomerase suppresses the progression of cervical cancer. EBioMedicine 41:408-419.
13. Wu X, Zhang L, Miao Y, Yang J, Wang X, Wang CC, Feng J*, Wang L* (2019) Homocysteine causes vascular endothelial dysfunction by disrupting endoplasmic reticulum redox homeostasis. Redox Biol 20: 46-59.
14. Zhang J, Zhu Q, Wang Xe, Yu J, Chen X, Wang J, Wang X, Xiao J, Wang CC, Wang L* (2018) Secretory kinase Fam20C tunes endoplasmic reticulum redox state via phosphorylation of Ero1α. EMBO J 37: e98699.
15. Fang J, Yang J, Wu X, Zhang G, Li T, Wang Xe, Zhang H, Wang CC, Liu GH*, Wang L* (2018) Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 17: e12765.
16. Li H, Yang K, Wang W, Niu Y, Li J, Dong Y, Liu Y, Wang CC, Wang L*, Liang H* (2018) Crystal and solution structures of human protein disulfide isomerase-like protein of the testis (PDILT) provide insight into its chaperone activity. J Biol Chem 293, 1192-1202.
17. Fan F, Zhang Y, Wang S, Han Y, Wang L*, Lu D* (2018) Characterization of the oxidative protein folding activity of a unique plant oxidoreductase, Arabidopsis protein disulfide isomerase-11. Biochem Biophys Res Commun 495:1041-1047.
18. Niu Y, Zhang L, Yu J, Wang CC* and Wang L* (2016) Novel roles of the non-catalytic elements of yeast protein-disulfide isomerase in its interplay with endoplasmic reticulum oxidoreductin 1. J Biol Chem 291:8283-8294.
19. Zhang L, Niu Y, Zhu L, Fang J, Wang Xe, Wang L* and Wang CC* (2014) Different interaction modes for protein-disulfide isomerase (PDI) as an efficient regulator and a specific substrate of endoplasmic reticulum oxidoreductin-1α (Ero1α). J Biol Chem 289:31188-31199.
20. Wang L*, Zhang L, Niu Y, Sitia R and Wang CC* (2014) Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1α to promote oxidative protein folding. Antioxid Redox Signal 20: 545-556.
21. Wang L, Zhu L and Wang CC (2011) The Endoplasmic Reticulum Sulphydryl Oxidase Ero1β Drives Efficient Oxidative Protein Folding with Loose Regulation. Biochem J 434:113-121.
22. Wang L, Li SJ, Sidhu A, Zhu L, Liang Y, Freedman RB, and Wang CC* (2009) Reconstitution of human Ero1-Lα/protein disulfide isomerase oxidative folding pathway in vitro: position-dependent differences in role between the a and a' domains of protein disulfide isomerase. J Biol Chem 284: 199-206.
综述 & 评论 (*责任作者)
1. Cheng F, Wang L* (2024) Slowing down the central dogma rate for alleviating aging. Innovation Life 2: 100049.
2. 胡昕炜, 王志珍, 王磊* (2023)"后AlphaFold时代"的蛋白质折叠问题. 《科学通报》 68: 2943-2950.
3. Wang L*, Wang CC (2023) Oxidative protein folding fidelity and redoxtasis in the endoplasmic reticulum. Trends Biochem Sci 48: 40-52.
4. Wang L*, Yu J, Wang CC (2021) Protein disulfide isomerase is regulated in multiple ways: Consequences for conformation, activities and pathophysiological functions. BioEssays 43: e2000147.
5. Wang L, Wang X, Wang CC* (2015) Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radical Bio Med 83:305-313.
6. 王曦, 王磊, 陈畅, 王志珍 (2014) 未折叠蛋白响应: 2014年拉斯克基础医学研究奖解读. 《科学通报》59: 3533-3534.
全部发表论文:https://scholar.google.com/citations?hl=en&user=NUni5KMAAAAJ
ORCID: 0000-0002-5071-5800
(资料来源:王磊研究员,2024-02-02)
王磊 博士 研究员
研究方向:内质网氧化还原稳态与人类健康
电子邮件:wanglei@ibp.ac.cn
电 话:010-64888501
通讯地址:北京市朝阳区大屯路15号(100101)
英文版个人网页:http://english.ibp.cas.cn/sourcedb/rck/EN_xsszmW/202303/t20230324_341380.html